9 STATES OF MATTER
- Batch(2k17),
Deptt. of Chemical Engg.
- BIT Sindri, Dhanbad
States
of matter. There are several states of matter but we hardly knew only 3-4
states i.e. Solid, Liquid, Gas and more on Plasma.
But
what about the other states of matter?
Let’s gain some knowledge about the rest states of matter namely:
1. BOSE-EINSTEIN CONDENSATE
2. QUARK-GLUON PLASMA (QGP)
3. QUANTUM LIQUID SPIN
4. DEGENERATE STATE
5. SUPERIONIC ICE
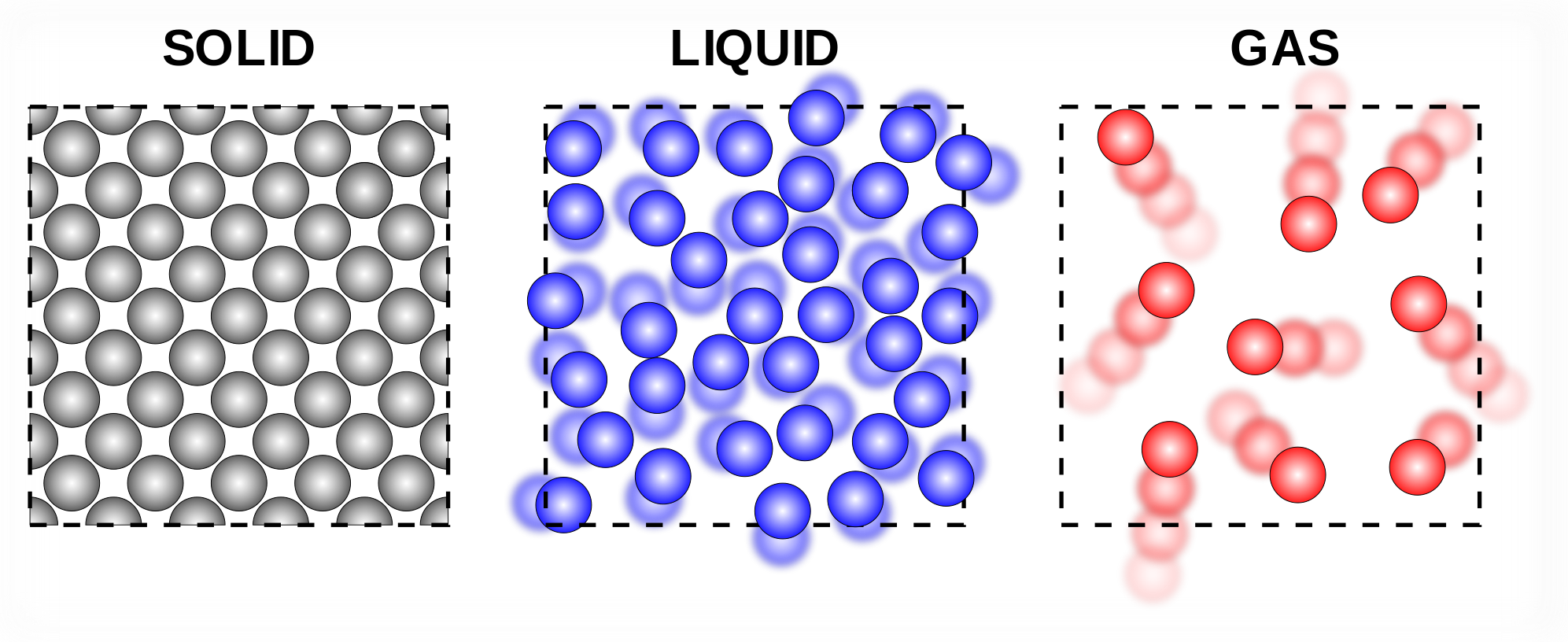
SOLID
Solid is one of the three
main states of matter, along with liquid and gas.
In a Solid, particles are packed tightly together so they
don't move much. The electrons of each atom are constantly in motion, so the
atoms have a small vibration, but they are fixed in their position. Because of
this, particles in a solid have very low kinetic energy.
Solids
have a definite shape, as well as mass and volume, and do not conform to the
shape of the container in which they are placed. Solids also have a high
density, meaning that the particles are tightly packed together.
Solids
are generally divided into three broad classes—crystalline, noncrystalline (amorphous), and quasicrystalline.
LIQUID
The liquid state of matter is an intermediate phase between solid and gas. Like the particles of a solid, particles in a liquid
are subject to intermolecular attraction; however, liquid particles have more
space between them, so they are not fixed in position. The attraction between
the particles in a liquid keeps the volume of the liquid constant. Liquids consist of atoms or molecules that are connected by
intermolecular bonds.
GAS
A gas is defined as
a state of matter consisting of particles that have neither
a defined volume nor defined shape. Gases are the phase of matter in which particles are usually
very far apart from one another, move very quickly, and aren't particularly
attracted to one another. Because the molecules in a gas are so far apart from
one another, gases are much less dense than liquids or solids.
Plasma is defined as a state of matter predominantly
comprised of ions and electrons. An ion is formed when an atom or molecule
gains or loses electrons, yielding an overall charge (either positive or
negative). The presence of charged ions means that a plasma is highly
electrically conductive and responds strongly to magnetic and electric
fields. Its behaviour is most comparable to that of a gas, as the plasma
has no defined volume but instead assumes the volume of the container it is in.
Despite all of the constituent particles being charged, typically the plasma
itself has no overall charge. However, some plasmas (non-neutral) can be
created with an overall charge (either positive or negative) and are composed
of pure electron, ion, positron, or antiproton plasmas.
Plasma consists of highly charged particles with
extremely high kinetic energy. The noble gases (helium, neon,
argon, krypton, xenon and radon) are often used to make glowing signs by using
electricity to ionize them to the plasma state.

BOSE-EINSTEIN CONDENSATE
Bose-Einstein condensates (BECs) - the existence of which
was predicted by Albert Einstein and Indian mathematician Satyendra Nath Bose
almost a century ago - are formed when atoms of certain elements are cooled to
near absolute zero (0 Kelvin, minus 273.15 Celsius).
At this point, the atoms become a single entity with
quantum properties, wherein each particle also functions as a wave of matter. BECs
straddle the line between the macroscopic world governed by forces such as
gravity and the microscopic plane, ruled by quantum mechanics.
This state exists by cooling a sample of rubidium to within a few degrees of
absolute zero. At this extremely low temperature, molecular motion comes very
close to stopping. Since there is almost no kinetic energy being transferred
from one atom to another, the atoms begin to clump together. There are no
longer thousands of separate atoms, just one "super atom."
Light appears to slow down as it passes through a
BEC, allowing scientists to study the particle/wave paradox. A BEC also
has many of the properties of a superfluid, or a fluid that flows
without friction. BECs are also used to simulate conditions that might exist in
black holes.
QUARK-GLUON PLASMA (QGP)
For a few millionths of a second after the Big Bang,
the universe consisted of a hot soup of elementary particles called quarks
and gluons. A few microseconds later, those particles began cooling to form
protons and neutrons, the building blocks of matter. The only place in the
universe where QGP exists is inside high-speed accelerators, for the
briefest flashes of time.
Systems consisting
of deconfined quarks and gluons, the fundamental constituents of matter and the
mediators of the strong force, are produced in controlled laboratory conditions
in reactions of heavy nuclei at ultra-relativistic energies. These so-called “the
quark-gluon plasmas” (QGP) exist at very high temperatures and energy
densities similar to those found a few microseconds after the Big Bang. The
quest to discover and characterize the properties of this new state of matter
via ultra-relativistic collisions of large nuclei is an active research thrust
at many experimental facilities such as the Bevalac, the CERN Super Proton
Synchrotron (SPS), the Relativistic Heavy Ion Collider (RHIC) and the Large
Hadron Collider (LHC).
Scientists creating QGP by smashing gold atoms together at
nearly the speed of light. These collisions can produce temperatures up to 4
trillion degrees — 250,000 times warmer than the sun’s interior and hot
enough to melt protons and neutrons into quarks and gluons.
The resulting super-hot, super-dense blob of matter, about a
trillionth of a centimeter across, could give scientists new insights into the
properties of the very early universe. So far, they have already made the
surprising discovery that QGP is a nearly frictionless liquid.

QUANTUM LIQUID SPIN
Spin liquids are quantum phases of matter that exhibit a variety of
novel features associated with their topological character. These include
various forms of fractionalization - elementary excitations that behave as
fractions of an electron. While there is not yet entirely convincing experimental
evidence that any particular material has a spin liquid ground state, in the
past few years, increasing evidence has accumulated for a number of materials
suggesting that they have characteristics strongly reminiscent of those
expected for a quantum spin liquid.
Quantum spin liquids are frequently
found in a class of materials known as frustrated magnets. In a conventional magnet, the interactions between spins result in
stable formations, known as their long-range order, in which the magnetic directions of
each individual electron is aligned.
In a frustrated magnet, the arrangement of electron spins prevents
them from forming an ordered alignment, and so they collapse into a
fluctuating, liquid-like state. In a true quantum spin liquid, the electron
spins never align, and continue to fluctuate even at the very lowest temperatures
of absolute zero, at which the spins in other magnetic states of matter would
have already frozen.
The conditions required for a quantum spin liquid
to form are often found in nature. The most famous example is the copper-based
mineral Herbertsmithite, for which there is
significant evidence to suggest that a quantum spin liquid state exists within
the frustrated magnetic layers of copper ions that make up its structure.
In experiments using neutron spectroscopy, the team revealed that α-RuCl₃ realises something extremely close to a special flavour of quantum
spin liquid called a Kitaev spin-liquid. A prerequisite for this particular quantum spin liquid
state is that the spins of the magnetic ruthenium ions form a frustrated
honeycomb network: a layered, two-dimensional hexagonal structure, similar to
that assumed by carbon atoms in graphite.

DEGENERATE STATE
Under extreme pressures,
such as those that occur within the nucleus of some stars, the particles are
compressed into a minimum space. Since two particles cannot occupy the same
space at the same moment, this causes the atoms to degenerate and lose
their structure: the electrons leave their orbits and begin to move at speeds
closer and closer to that of light, exerting an expansive force
that compensates for the external pressure.
If this continues to increase and exceeds the so-called Chandrasekar limit, then the external pressure becomes untenable and the atomic nuclei also degenerate, losing their structure, collapsing into an accumulation of neutrons and protons.
SUPERIONIC ICE
Scientists
first predicted in 1988 that water would transition to an exotic state of
matter characterized by the coexistence of a solid lattice of oxygen and
liquid-like hydrogen - a phase called superionic ice – when subjected to
the extreme pressures and temperatures that exist in the interiors of
water-rich giant planets like Uranus and Neptune.
Using laser-driven
shockwaves to flash-freeze water into its exotic superionic phase
and in-situ x-ray diffraction, they observed the nucleation of a
crystalline lattice of oxygens in a few billionths of a second, revealing for
the first time the microscopic structure of superionic ice.
Water as a beginning and as an end. Water is the only substance present
in nature in the three classical states, and it is also the substance in which,
at the beginning of 2018, a new form or state of arrangement was discovered:
superionic ice. To achieve this, ice crystals were subjected to a pressure 2
million times higher than atmospheric pressure and at a temperature close to
5,000°C. That brutal pressure forces
the ice to adopt a very compact packing, but, at the same time,
the high temperature dissolves the bonds of the water molecule. The result is
that in superionic ice two phases coexist: one liquid and one solid. Oxygen
atoms adopt a crystalline structure, through which hydrogen nuclei
flow.

FACTS TO KNOW…
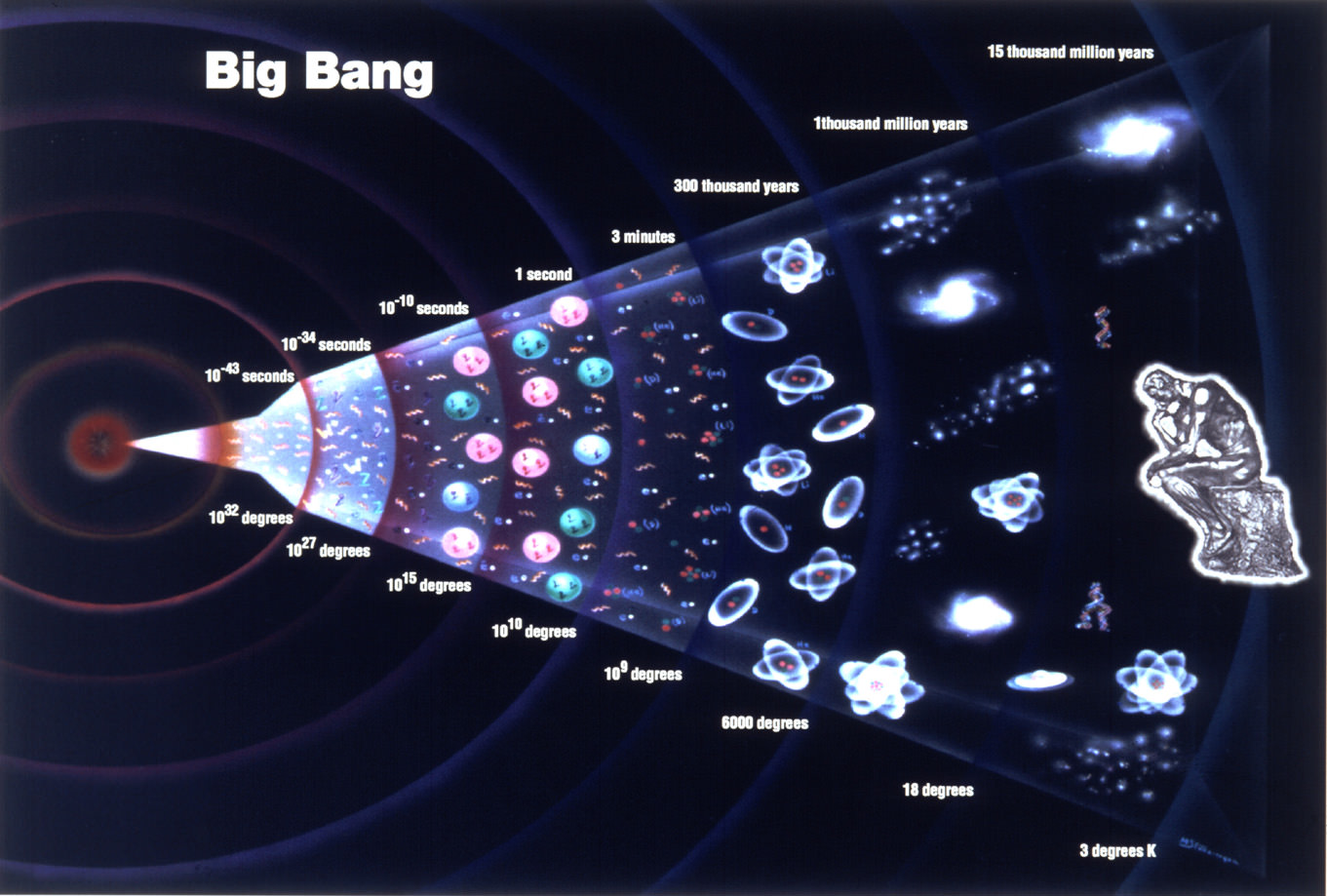
WHERE DOES MATTER COME FROM?
All matter in the Universe was
created by the Big Bang, 14 billion years ago. In less than a second,
the Universe was filled with vast amounts of energy, such as light and heat.
The explosion made the Universe expand. As it expanded, it cooled, and particles
with mass formed and clumped together.
BIG
BANG
The Universe is
still expanding and cooling today. As it cools, the force of gravity
draws floating particles of matter together to form new stars and galaxies.


5 comments
Click here for commentsGood to know about the existing unknown matters around us. Much informative.
ReplyVery informative, I was not aware 😀
ReplyGlad to know about the nine states of matter, well explained and arranged here. Keep sharing! Kudos!
ReplyVery informative and presented in a very simple way...
ReplyThank you everyone for your positive response. I really appreciate it.
ReplyConversionConversion EmoticonEmoticon